We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
The DYKDDDDK epitope
Purification is a common issue when producing recombinant proteins in vitro. In order to discriminate them from other components of the production medium, the most used way is to fuse them with a polypeptidic partner, or tag, which will be bound specifically upon purification. Poly-arginine, in combination with a cation exchange-based purification method, or poly-histidine, in combination with an immobilized metal ion (such as Ni2+) affinity chromatography, have been widely used tags.
Epitope tags provide double advantage as they allow both immuno-affinity purification of recombinant proteins and their staining within a transformed cell (e.g. by immunofluorescence, provided the epitope tag is exposed on the protein surface).
The DYKDDDDK epitope has been specifically engineered to provide recombinant proteins an increased sensitivity for their detection as well as their purification when using antibodies which are specific for this octapeptide. Moreover, the presence of enterokinase cleavage site (Asp-Asp-Asp-Asp-Lys) included within the epitope provides a convenient way to subsequently recover native proteins.
Our highly specific anti-DYKDDDDK epitope antibodies clone FG4R and clone L5 have been developed to show less background as well as more sensitivity than the standard M2 clone.
Higher signal-to-noise ratio
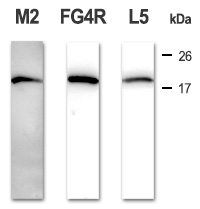
After transferring proteins onto the nitrocellulose, the membrane underwent low saturation conditions: incubation overnight at +4°C with 0,5% low-fat milk in TBS instead of standard 5% (see the standard conditions).
Under these conditions, the standard M2 clone shows a high unspecific background signal, whereas our two clones FG4R and L5 show none. Exposure: 20 sec.
Higher sensitivity
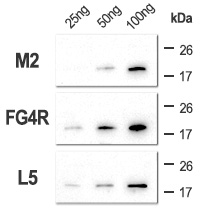
Various amounts of sample were resolved by SDS-PAGE, from 100 ng of pure sample to as low as 2 ng, to check the sensitivity of the primary antibodies.
Although the standard M2 clone does not detect 25 ng of pure sample, our two clones still show visible signal for this conditions. Lower amounts (10 ng, 5 ng and 2 ng) were not detected (data not shown). Exposure: 30 sec..
High specificity
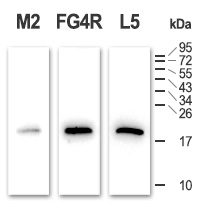
100 ng of pure sample were mixed with 100 µg of whole cell lysate (cells were lysed using standard RIPA buffer), and resolved by SDS-PAGE to check the specificity of the antibodies for the DYKDDDDK epitope.
The blot shows no other visible signal than the expected sample, meaning the primary antibodies are highly specific for the octapeptide. Exposure: 40 sec.
Standard conditions
Sample:
Migration:
FLAG®-tagged partial TNF-α (residues 85-223) samples were resolved by SDS-PAGE (17% acrylamide) under denaturing conditions (DTT & β-mercaptoethanol) after 1 min heating in boiling water.
Samples were separated using a Tris-Glycine buffer under a constant voltage applied for 30 min at 80V and then for 2 hrs at 100V at room temperature.
Transfer:
Saturation:
Proteins were transferred onto a nitrocellulose membrane under a constant voltage applied for 1 hr at 100V at 4°C in a Tris-Glycine-Methanol buffer.
The membrane was incubated in 5% low-fat milk / TBS for 1 hr at 37°C.
Primary antibody staining:
M2: mouse anti-FLAG® epitope antibody, clone M2.
FG4R: mouse anti-DYKDDDDK epitope antibody, clone FG4R, from Covalab (Cat# mab90006-P).
L5: rat anti-DYKDDDDK epitope antibody, clone L5, from Covalab (Cat# mab50778).
Antibodies were diluted 1:1000 in 1% low-fat milk / TBS-T. Membranes were incubated overnight at 4°C, and washed 3 x 5 min with TBS-T.
Secondary antibody staining:
Clones M2 & FG4R: HRP-conjugated goat anti-mouse IgG (H+L) antibody from Covalab (Cat# lab0252).
Clone L5: HRP-conjugated rabbit anti-rat IgG (H+L) antibody from Covalab (Cat# lab0289).
Antibodies were diluted 1:2000 in 1% low-fat milk / TBS-T. Membranes were incubated for 1 hr at 37°C, and washed 3 x 5 min with TBS-T.
Revelation:
Membranes were incubated with Covalight® enhanced chemiluminescence reagent according to manufacturer’s instructions, and exposed under a CCD camera for various amounts of time (see figures).