We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
 CHIMERIZATION - HUMANIZATION
CHIMERIZATION - HUMANIZATION
Covalab has developed a robust mAb engineering platform including mAb chimerization, mAb humanization, Fc optimisation and ADC to increase the mAb efficacy. The complete mastery of mAb chimerization and humanization thereby reduces immunogenicity, increases effector action and in vivo survival. A wide base of knowledge has been acquired through each humanization project, demonstrating that certain residues can be critical for affinity and/or for mAb potency.
Covalab’s molecular biology & immunology expertise allows to engineer murine mAbs as humanized mAbs keeping the key properties of the parental murine mAbs and bringing in critical improvements regarding mAb potency.
 |
 |
 |
|
Murine antibody 100% mouse -omab |
Chimeric antibody > 67% human -ximab |
Humanized antibody > 94% human -zumab |
 Covalab mAb humanization approach
Covalab mAb humanization approach
The majority of therapeutic mAb on the market originate from mouse hybridomas, but these have been engineered by the humanization process to overcome therapeutic deficiencies of mouse mAbs.
A humanization platform has been generated at Covalab based on some strategically approaches related to molecular biology, mAb authenticity analysis and mAb expression as described above.
 Different complementary strategies humanization
Different complementary strategies humanization
CDR Grafting |
SDR Grafting |
|
Humanized mAbs are constructed following selection of human acceptor mAb (source IMGT) selected based on CDR and FR homology (CDR grafting). This technique consists in replacing the CDRs of a human antibody by the CDR regions of the murine antibody of interest. This is a commonly employed technique, which was introduced in 1986 by Jones. The “grafting” of the CDR on a human acceptor mAb can also lead to a significant reduction or complete loss of binding affinity, because certain framework residues are important for maintaining the conformation of the CDRs or are even directly involved in antigen binding. This problem could be solved by reintroducing murine residues into the human framework at positions that are deemed to be critical for CDR loop (backmutation step). |
CDR-grafted humanized mAbs may still evoke « anti V region responses » when administered in patients. To minimize « anti V region responses », the mAb may be humanized by grafting onto the human templates only the specificity determining residues (SDRs), the residues that are essential for the surface complementarity of the mAbs and its antigen. According Padlan (1994) from 20 to 33% of the residues of the CDR regions are required for binding with the antigen, so the SDR grafting technique (specificity-determining residues) consists in introducing the murine SDR in human FR regions, in order to having a theoretical decreased immunogenicity relative to a CDR grafting approach. |
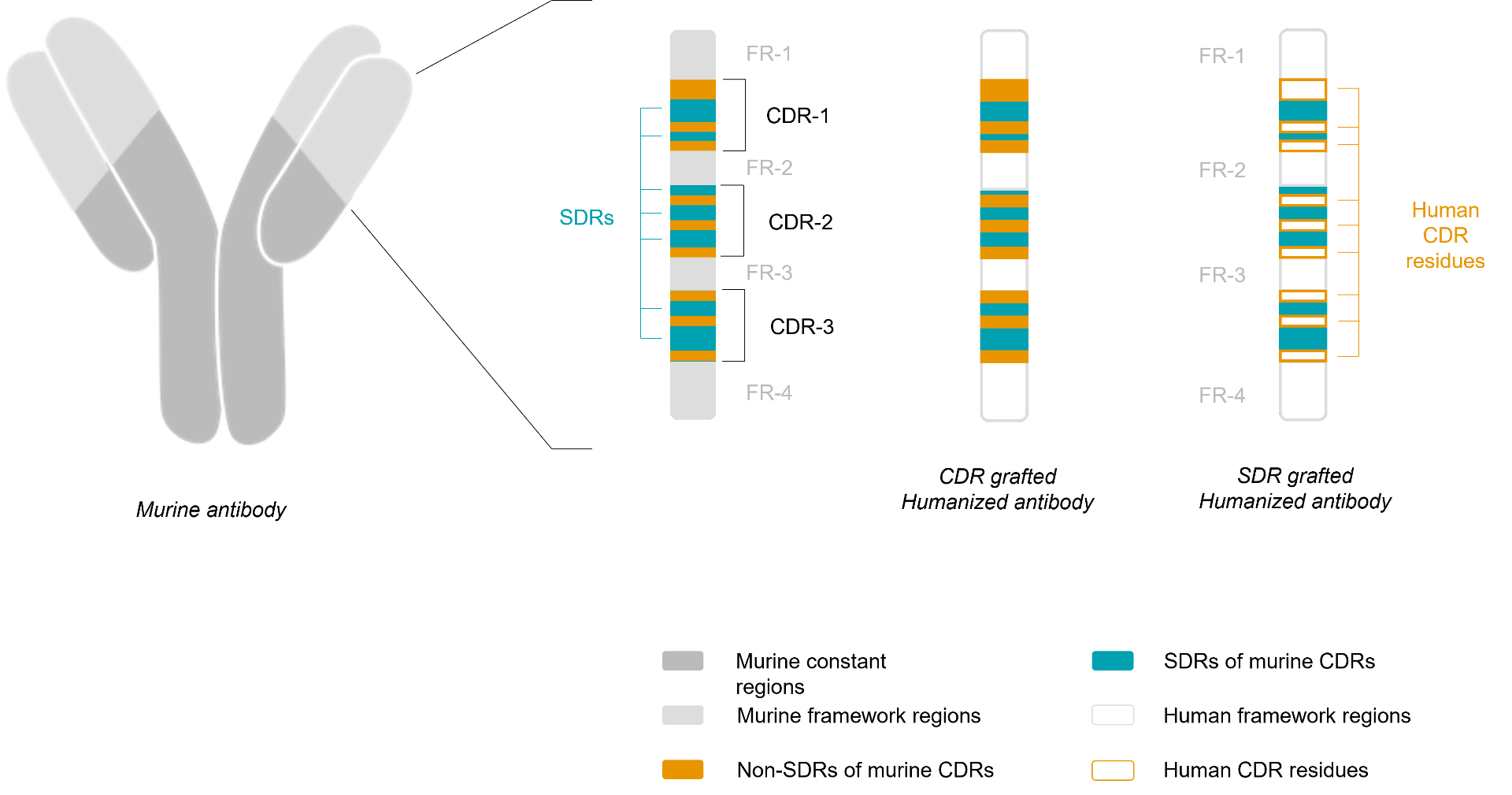
Typically mAb humanization, whether by CDR or SDR grafting, involves the use of a single human template for the enture VL or VH domain. The homology between the human template sequences and mAb to be humanized may be maximized by using templates from multiple human germline sequences corresponding to the different segments of the variable domain.
 Protocol overview
Protocol overview
The entire procedure followed by Covalab for the humanization of murine mAbs is broadly outlined below.
| ≈ 2 weeks |
1st generation ≈ 2 months |
2nd generation ≈ 2 months |
 |
 |
 |
Chimerization |
CDR Grafting |
BackmutationSDR Grafting |
|
Antibody sequencing from the hybridoma |
Search the human backbone (FR region)
Sequence design
Gene synthesis & expression vector construction
Validation of expression vector by transient transfection in CHO, ELISA and FACS analysis
Lead mAb purification |
Sequence design and gene synthesis in expression vector
Validation of expression vector by transient transfection in CHO, ELISA and FACS analysis
Lead mAb purification |
Go / No go step
Our team will works in close collaboration with you and discuss all along the project before moving on.
We decide together to take the next step according to the humanized % achieved.
 Learn more about humanization
Learn more about humanization
In 1975, the discovery of monoclonal antibody has to imagine that these proteins could be used with a high specificity for the treatment of disease and especially cancer.
In 1982, the first successful use of monoclonal antibodies in clinical were published. They hinted many opportunities for therapeutic use of monoclonal antibodies.
However, 12 years later one monoclonal antibody had received permission to clinical use. The main problem encountered on mAb was used in therapeutic due to their murine origin, the patient produces human anti-murine antibody (HAMA). These HAMA induce rapid elimination of mAb, as well as side effects. In fact, antibody engineering to transform murine Ab to human antibodies developed.
The first step was to convert them into chimeric antibodies, wherein the constant regions of heavy and light chains of the murine immunoglobulin are replaced by human constant regions. The first step to humanize murine Ab resulted in a significant decrease in immune responses. Between 1994 and 1998, several chimeric antibodies were able to be used clinically. However, the formation of human Ab against chimeric Ab (HACA) pushing the pharmaceutical industry to focus on the humanized antibodies.
The first humanization of antibodies has been described in 1986 and consists of producing human variable regions near regions, without losing the humanized antibody its specificity and affinity of the murine antibody. Humanization (Ab having 90% human sequence) corresponds to the grafting of the hypervariable portions (CDR) of a murine Ab on a human immunoglobulin. These humanized antibodies comprise only a small fraction of the Ab mouse (10%): they are theoretically better tolerated by the human organism.
The ultimate goal of the humanization of an antibody is to produce variable regions near human regions without losing the humanized antibody affinity, specificity of murine and functionalities (PCD, CDC, ADCC antibodies...).
To achieve this, it must be transferred into a human framework "acceptor" (human FR region) amino acids of the CDRs from the murine monoclonal antibody "donor". This concept relies on the fact that the six CDR regions of the variable regions of the heavy and light chains contain a majority of amino acids comprising the binding site of the antigen.
