We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
 ANTIBODY FRAGMENTATION SERVICE
ANTIBODY FRAGMENTATION SERVICE

Distinct fragments of the immunoglobulin can be generated by cleaving the molecule using proteases.
Targeting the hinge region allows to generate two types of fragments of interest depending on the final application:
- Antigen-binding fragments such as Fab and F(ab')2
- Class-defining fragments such as Fc which do not bind antigen
Depending on which side of the hinge region (N- or C-terminal) is targeted, the proteolytic cleavage produces either two Fab and one Fc fragment or one F(ab')2 fragment.
 PAPAÏN FRAGMENTATION
PAPAÏN FRAGMENTATION
When immunoglobulins are incubated with papain in the presence of a reducing agent, several peptide bonds above the hinge region are hydrolyzed, producing three fragments of similar size (50kDa):
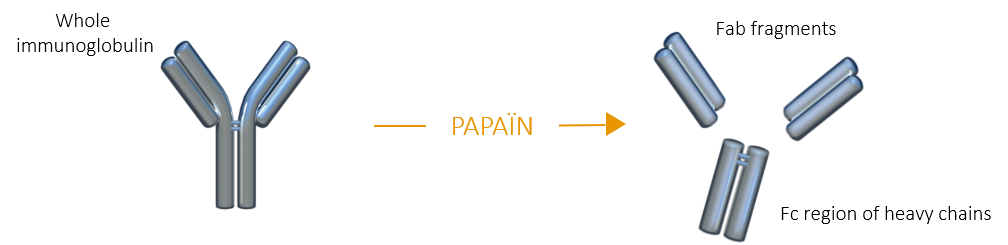
Fab fragment |
Fc fragment |
|
Compared to whole IgG, Fab fragments can penetratecells and tissues and bind antigens more easily thanks to their lower steric hindrance. Moreover, the absence of Fc region prevents Fab fragments from nonspecific binding to Fc receptors. |
When the Fc fragment is of interest, papain is consequently the enzyme of choice. Papain is primarily used to generate Fab fragments, but it can also be used to generate F(ab’)2 fragments when used in specific physico-chemical conditions. |
 PEPSIN FRAGMENTATION
PEPSIN FRAGMENTATION
Digestion of immunoglobulins by pepsin occurs below the hinge region of the heavy chains, resulting in a F(ab’)2 fragment which is composed of two Fab fragments still linked together by the remaining portion of their respective heavy chains at the hinge region. The Fc fragment is extensively degraded, and its small fragments can be separated from F(ab')2 by dialysis, gel filtration or ion exchange chromatography.
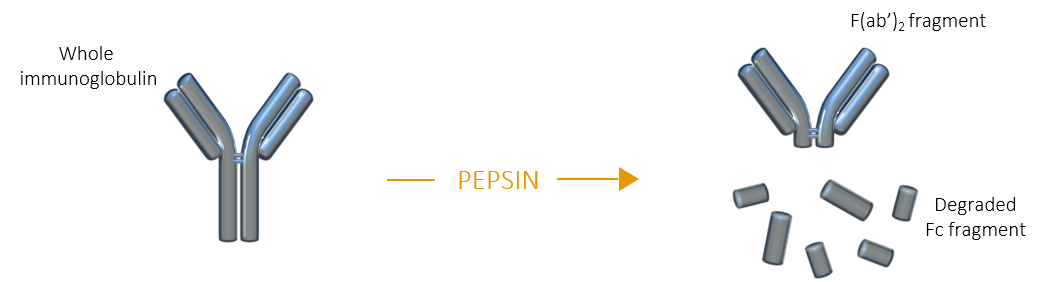
F(ab')2 fragment |
| F(ab')2 fragments share many advantages with Fab fragments, but unlike the latter, F(ab’)2 fragments remain divalent and can be used to bind and precipitate antigens |
