We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
 COVISOLINK® (PATENT WO2015097267A1)
COVISOLINK® (PATENT WO2015097267A1)
A proprietary technology allowing the grafting of antibody and antibody fragments on specific site
Monoclonal antibodies coupled to highly toxic agents or ADC (Antibody-Drug Conjugate) are becoming a significant component of anticancer treatment. A major aim of Covalab is the optimization and development of therapeutic monoclonal antibodies in oncology. The results expected with this project are far-reaching since this technology is potentially applicable to a large variety of antitumor (or anti-stromal) antibodies since it is not restricted by antigen specificity.
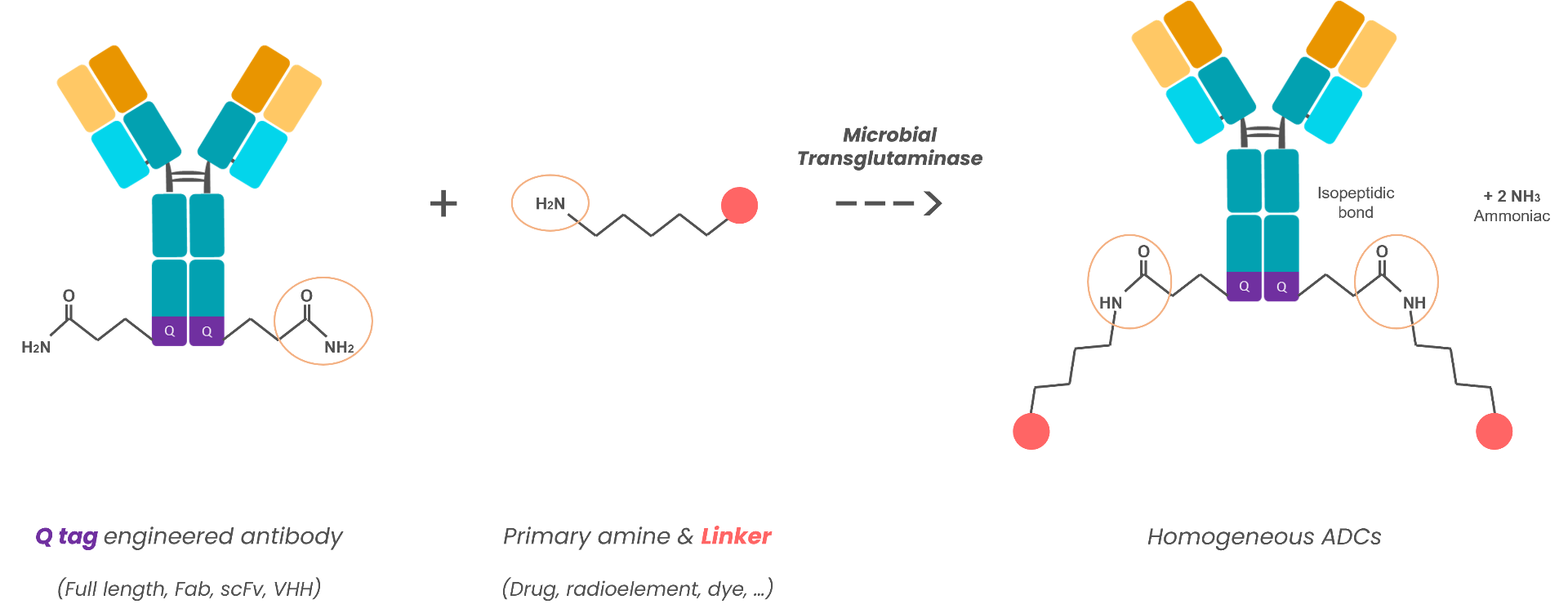
Currently approved immunoconjugates are heterogeneous in terms of degree of substitution, which is suboptimal both in terms of antitumor efficacy and risk of toxicity. The Covlsolink (Covalently Isopeptide Crosslinking) technology by Covalab allows the precise degree of substitution, through introduction in the IgG gene by genetic engeneering of specific sites allow enzymatic ADC covalent coupling. The target being a ratio of 2 conjugate molecules for each molecule of antibody, which appears to be the best compromise in terms of amount of drug delivered to the tumor cell and optimized kinetics of the immunoconjugate in the body.
|
APPLICATIONS |
||
|
THERAPEUTIC ADC Radiotherapy |
DIAGNOSTIC Radioimaging Immunodiagnostic |
R&D Labelling Conjugation |
 A platform for ADC generation
A platform for ADC generation
CovIsoLink™ technology is used to develop new antibody drug conjugates (ADCs) with uniform stoichiometry by controlling: the location of coupling sites on the antibody without affecting its immunoreactivity and the number of molecules coupled per molecule of antibody by controlling the coupling ratio. With our technology, we have the ability to control the drug antibody ratio (DAR) and consequently the toxicity and efficacy of therapeutic molecules. The results expected with this project are far-reaching since this technology is potentially applicable to a large variety of antitumor (or anti-stromal) antibodies since it is not restricted by antigen specificity.
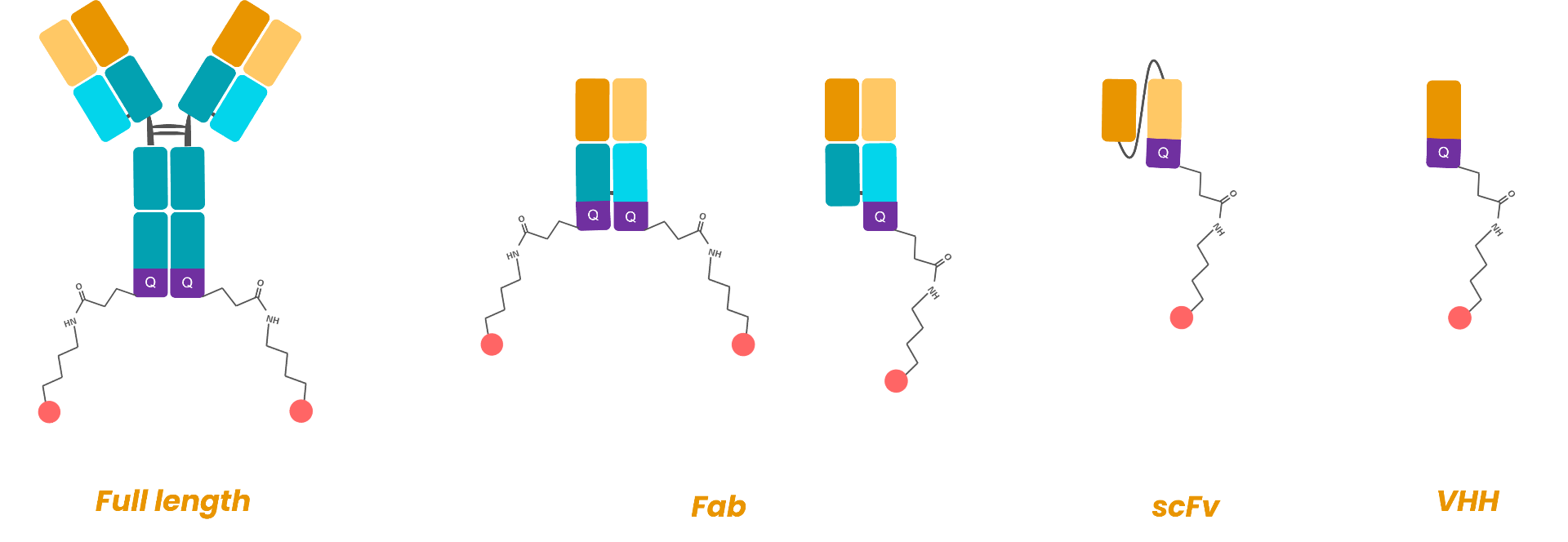
Suitable to all mAB format (full length, Fab, scFv, VHH)
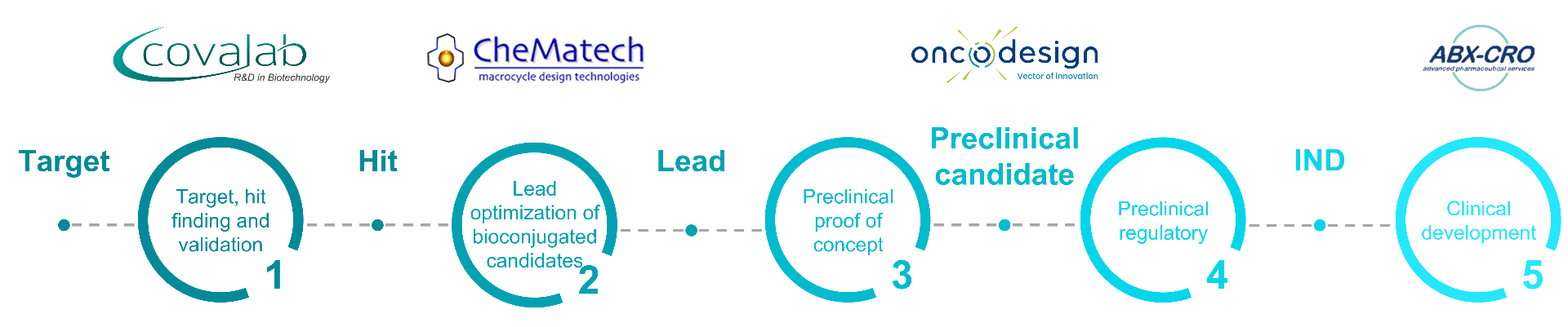
 A consortium for your radiopharmaceutical projects
A consortium for your radiopharmaceutical projects
Covalab with its partners Oncodesign, CheMatech and ABX-CRO have put their expertise together in order to offer the solution Drive MRT (DRug Integrative discoVEry of Molecular RadioTherapy) for drug discovery support. We can assist on all steps for your radiopharmaceutical projects thanks to our own competences, from hit finding, lead optimization to the preclinical candidate drug generation.
- Target validation
- DNA immunization / protein / hapten
- Selection of the best antibody format (Fab, ScFv, VHH) from naive or immune bank
- Selection and validation of antibodies
- Production at small scale, purification and validation
- Targeted bioconjugaison
- Screening of in vitro compounts
- Bioconjugaison strategy
- Chelating agents optimization
- Linker optimization
- Biochemistry and biology in vitro
- In vitro and in vivo DMPK
- Radiolabelling
- Biodistribution by nuclear imaging
Visit Chematech website
- In vitro and in vivo DMPK
- In vivo pharmacology
- Efficiency proof of concept
- Pharmaco-imaging
- Time activity curve
- Study of combination with reference's drugs
- Formulation
- Premature estimation of the toxicity
Visit Oncodesign website
- Project management and regulatory filing
- BPF synthesis / Scale up
- Radiopharmaceutical development
- GLP bioanalysis and pharmacokinetic
- Dosimetry
- Regulatory studies of toxicity
Visit Oncodesign website
- Clinical project management
- GLP radiolabelling
- Dosimetry (QDOSE®)
- Data management and biostatistics
- Medical report
- Medical surveillance
- Pharmacovigilance
Visit ABX-CRO website
 A proprietary technology
A proprietary technology
Since 2013, Covalab has filed a international patent (n°WO2015097267A1).
This program was awarded CLARA in 2015 and 2017, ANR in 2017 and the European program EUROSTARS 2015.
|
|
|
|






